Ischemic Stroke
Author Credentials
Author: Cynthia Leung MD PhD, Assistant Clinical Professor, The Ohio State University College of Medicine.
Editor: Rahul Patwari, MD. Rush University, Chicago, Illinois.
Last Update: 2015
Introduction
Stroke is the fourth leading cause of death and the leading cause of disability in the US with estimated direct and indirect costs of roughly 70 billion dollars per year. Based on current estimates, the prevalence of stroke is expected to increase by twenty percent by the year 2030. Advancements in the diagnosis and treatment of stroke must continue to compensate for the increasing stroke burden on our society. Stroke is characterized by the acute onset of neurologic deficit caused by disruption of cerebral blood flow to a localized region of the brain. The reversibility and extent of symptoms in stroke is critically dependent on the duration of this disruption. Therefore, early recognition and treatment is the key to reducing morbidity and mortality associated with stroke. As the first physician to see the patient with acute stroke, the actions of the Emergency Physician can have a profound impact on the outcome of stroke patients. Acute stroke most commonly results from occlusion of an intracranial artery by thrombosis within the artery, thromboembolism from an extra-cranial source, or hemorrhage. Eighty seven percent of strokes are ischemic in etiology with the remainder caused by intracerebral or subarachnoid hemorrhage. This module will focus exclusively on the evaluation and treatment of acute ischemic stroke. The evaluation and treatment of hemorrhagic stroke can be found in the intracranial hemorrhage module. Patients with stroke may present with a variety of neurologic symptoms including changes in vision, changes in speech, focal numbness or weakness, disequilibrium or alteration in level of consciousness. There are many alternate diagnoses that can mimic the symptoms of stroke. The differential diagnosis includes:
- Structural brain lesion (tumor, AVM, aneurysm, hemorrhage)
- Infection (cerebral abscess, septic emboli)
- Seizure Disorder and post-seizure neurologic deficit (Todd’s paralysis)
- Peripheral Neuropathy (Bell’s palsy)
- Complicated Migraine
- Hypoglycemia
- Conversion Disorder
Objectives
- Recognize signs and symptoms of stroke
- Identify clinical features suggestive of common stroke mimics
- Describe the initial management of acute stroke
- List indications and contraindications for thrombolytic therapy
Initial Actions and Primary Survey
The initial actions in the evaluation of a patient with suspected stroke begin with emergent stabilization of the patient. As with any emergent patient, the primary survey includes assessment of the patient’s airway, breathing and circulation. Hypoxemia and hypotension due to stroke or co-morbid conditions may worsen stroke symptoms and lead to death. Therefore, treatment of any critical conditions found on primary survey must be initiated prior to continuing the evaluation. Next a focused H&P is performed to assess level of neurologic dysfunction, exclude alternate diagnoses, and determine the patient’s eligibility for therapy.
Presentation
The initial diagnosis of acute stroke is based on clinical findings. Part of the challenge in making the diagnosis is that there is no “textbook” presentation of stroke. The signs and symptoms of stroke are highly variable and depend not only on the particular blood vessel occluded, but also the extent of occlusion and amount of circulation provided by collateral vessels. Presentations may vary from multiple profound neurologic deficits in a large vessel occlusion to very subtle isolated deficits when smaller vessels are occluded.
History
The single most important component of the history is the exact time of onset of symptoms. This is defined as the time when the patient was last known to be symptom-free, commonly referred to as the “last known well”. In cases where the patient’s last known well time is unclear, focused questions should be deployed to help narrow down the time window as much as possible. For example, if the patient awakens from sleep with symptoms, questioning the patient about waking in the middle of the night to walk to the restroom or kitchen may help to determine a more accurate last known well time. In patients who were awake during symptom onset, asking about specific activities such as phone calls or television shows may help to further focus the timeframe of onset. Friends and family should also be asked to provide collateral information when possible. The remainder of the history should focus on factors which may help differentiate a stroke mimic from a true stroke. The HPI should include a detailed history of the onset, time course and pattern of symptoms to help distinguish between stroke and alternate diagnoses. Symptoms which achieve maximal intensity within seconds to minutes of onset and simultaneously affect multiple different systems at once are typical of stroke. In contrast, symptoms which progress slowly over time or progress from one area of the body to another are more suggestive of stroke mimic. The past medical history should include assessment of stroke risk factors as well as risk factors for stroke mimics. Stroke risk factors include hypertension, diabetes, hyperlipidemia, tobacco abuse, advanced age, atrial fibrillation or prosthetic heart valve, and prior stroke. In patients receiving thrombolytic therapy, the most common stroke mimics include complicated migraine, seizure and conversion disorder. A past medical history which includes any of these disorders should heighten suspicion of these alternate diagnoses. The table below shows other clinical features suggestive of these stroke mimics.
| Structural | Non-structural |
|---|---|
| Tumor Abscess Bleeding (epidural, subdural hematoma) | Hypoglycemia Hypertensive encephalopathy Infection (encephalitis or meningitis) Middle ear pathology (Meniere’s disease or labryinthitis) Drug toxicity (e.g. phenytoin, lithium) Complicated migraine Postictal paralysis Demyelinating disease Bell’s Palsy |
Physical Exam
Once the primary survey is complete, a thorough neurologic exam should be performed. This should include assessment of level of consciousness, cranial nerves, strength, sensation, cerebellar function and gait. The NIH Stroke Scale provides a standardized clinical assessment which is generalizable from one physician to another and allows monitoring of the patients neurologic deficits over time.Stroke Scale Calculator can be found on MDcalc.com
| Level of Consciousness | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0: Alert | 1: Drowsy | 2: Stuporous | 3: Comatose | |||||||
| Level of Consciousness Questions: Age? Month? | ||||||||||
| 0: Both Right | 1: One Right | 2: None Right | ||||||||
| Level of Consciousness Commands: "Close Your Eyes" & "Make A Fist" | ||||||||||
| 0: Both Right | 1: One Right | 2: None Right | ||||||||
| Best Gaze | ||||||||||
| 0: Normal | 1: Partial gaze palsy | 2: Forced deviation | ||||||||
| Visual Fields | ||||||||||
| 0: No visual loss | 1: Parial hemianopia | 2: Complete hemianopia | 3: Bilateral hemianopia | |||||||
| Facial Paresis | ||||||||||
| 0: Normal movement | 1: Minor paresis | 2: Partial paresis | 3: Complete palsy | |||||||
| Best Motor (Left Arm) | ||||||||||
| 0: No Drift | 1: Drift | 2: Some effort vs gravity | 3: No effort vs gravity | 4: No movement | 9: Untestable | |||||
| Best Motor (Right Arm) | ||||||||||
| 0: No Drift | 1: Drift | 2: Some effort vs gravity | 3: No effort vs gravity | 4: No movement | 9: Untestable | |||||
| Best Motor (Left Leg) | ||||||||||
| 0: No Drift | 1: Drift | 2: Some effort vs gravity | 3: No effort vs gravity | 4: No movement | 9: Untestable | |||||
| Best Motor (Right Leg) | ||||||||||
| 0: No Drift | 1: Drift | 2: Some effort vs gravity | 3: No effort vs gravity | 4: No movement | 9: Untestable | |||||
| Limb Ataxia (Scored only if present) | ||||||||||
| 0: Absent | 1: Present in 1 limb | 2: Present in 2 limbs | ||||||||
| Sensory (Pinprick) | ||||||||||
| 0: Normal | 1: Partial loss | 2: Dense loss | ||||||||
| Best Langunage | ||||||||||
| 0: No Aphasia | 1: Mild to moderate aphasia | 2: Severe aphasia | 3: Mute | |||||||
| Dysarthria | ||||||||||
| 0: Normal articulation | 1: Mild to moderate dysarthria | 2: Unintelligible or worse | ||||||||
| Neglect/Inattention | ||||||||||
| 0: No neglect | 1: Partial neglect | 2: Complete neglect | ||||||||
| ||||||||||
Signs and symptoms of stroke should follow a vascular distribution of the brain. Knowledge of the functional areas supplied by each of the major intracranial blood vessels helps to predict signs and symptoms associated with occlusion of that particular vessel.
Common stroke syndromes
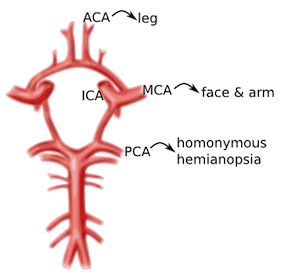
ACA: unilateral weakness and/or sensory loss of contralateral lower extremity greater than upper extremity MCA: unilateral weakness and/or sensory loss of contralateral face and upper extremity greater than lower extremity with either aphasia (if dominant hemisphere) or neglect (if non-dominant hemisphere) PCA: unilateral visual field deficit in both eyes (homonymous hemianopsia). Vertebrobasilar syndromes have multiple deficits which typically include contralateral weakness and/or sensory loss in combination with ipsilateral cranial nerve palsies. Suspicion for posterior circulation stroke is heightened if the patient exhibits one of these signs or symptoms beginning with “D”: diplopia, dysarthria, dysphagia, droopy face, dysequilibrium, dysmetria, and decreased level of consciousness. Nausea and vomiting are also frequently associated with brainstem stroke. Lacunar infarcts are small strokes (measuring less than 1.5 cm) caused by occlusion of one of the deep perforating arteries which supplies the subcortical structures and brainstem. Lacunar infarcts can produce a large variety of clinical deficits depending on their location within the brainstem and have been characterized by more than 70 different clinical syndromes. However, the vast majority of lacunar strokes are described by the 5 most common lacunar syndromes: pure motor hemiparesis, sensorimotor stroke, ataxic hemiparesis, pure sensory stroke, and dysarthria-clumsy hand syndrome.
Diagnostic Testing
Rapid evaluation of patients with suspected stroke is critical because there is a very narrow time window in which stroke patients are eligible for treatment. A panel of experts convened by the NINDS has established several critical events in the identification, evaluation, and treatment of stroke patients in the ED. Goals for time frames of this evaluation are summarized below: Guidelines for Initial Evaluation and Treatment of Acute Stroke in the Emergency Department
- Door to physician: 10 minutes
- Door to stroke team: 15 minutes
- Door to lab work completed: 45 minutes (CBC, BMP, PT/PTT, UA, EKG, CXR)
- Door to non-contrast CT-head ordered: 25 minutes
- Door to CT interpretation: 45 minutes
- Door to decision to give tPA: 45 minutes
- Door to drug administration: 60 minutes (and less than 3 hours from onset)
- Door to admission: 180 minutes
The diagnosis of stroke is based primarily on clinical presentation. The remainder of the diagnostic workup is focused on excluding alternative diagnoses, assessing for comorbid conditions and determining eligibility for therapy. The diagnostic workup includes:
Brain Imaging
Head CT without contrast should be performed on all patients to exclude intracranial hemorrhage. Unenhanced head CT is often able to identify other structural brain lesions and may detect early signs of stroke. Because radiologic changes associated with stroke are usually not visible on CT for several hours, the most common CT finding in acute ischemic stroke is normal brain. However, multiple subtle findings associated with acute ischemic stroke may be present in the first 3 hours after symptom onset. The earliest finding that may be seen on CT is hyperdensity representing acute thrombus or embolus in a major intracranial vessel. This is most frequently seen in the MCA (“hyperdense MCA sign”) and basilar arteries (“hyperdense basilar artery sign”). Subsequent findings include subtle hypoattentuation causing obscuration of the nuclei in the basal ganglia and loss of gray/white differentiation in the cortex. Frank hypodensity on CT is indicative of completed stroke and may be a contraindication to thrombolytic therapy (see below). At specialized stroke centers, alternative testing such as diffusion weighted MRI (DWI) or CT angiography/CT perfusion studies may also be performed as these modalities are more sensitive for detecting early or evolving infarct. However, the clinical utility in the ED setting is limited by relatively long duration of testing and lack of widespread availability.
Serum Glucose
Hypoglycemia may cause alteration in level of consciousness and any variety of neurologic symptoms. Point of care blood glucose level must be performed to exclude hypoglycemia prior to initiation of thrombolytic therapy.
EKG
EKG should be performed to exclude contemporaneous acute MI or atrial fibrillation as these conditions are frequently associated with thromboembolic stroke.
Additional laboratory studies
CBC, chemistries, PT/INR, aPTT, and cardiac markers are recommended to assess for serious comorbid conditions and aid in selection of therapy. However treatment should not be delayed for results of these additional studies unless a bleeding disorder is suspected.
Treatment
Intravenous Thrombolytic Therapy
The main goal of therapy in acute ischemic stroke is to remove occlusion from the involved vessel and restore blood flow to the affected area of brain. Intravenous recombinant Tissue Plasminogen Activator (rtPA) is currently the only FDA-approved therapy for acute ischemic stroke. rtPA is a fibrinolytic agent that catalyzes the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown. Treatment with IV rtPA has been shown to increase the percentage of patients with good functional outcome at 3 months and 1 year after stroke onset. rtPA has been FDA approved for use up to 3hrs after symptom onset. In addition, the American Heart Association has recommended rtPA for use up to 4.5 hours after symptom onset in a select subgroup of patients. Good functional outcomes are most likely to be achieved if rtPA is administered within 90 minutes of symptom onset. The likelihood of a good outcome decreases with increasing time from onset of symptoms. Therefore, every effort should be made to ensure that there are no delays in administration of thrombolytic therapy to eligible patients. The major complication of rtPA administration in stroke is symptomatic intracranial hemorrhage. Careful selection of patients with an appropriate risk/benefit ratio is imperative to reduce the risk of symptomatic ICH. Inclusion and exclusion criteria for intravenous rtPA therapy are shown in the table below: Inclusion and Exclusion Criteria for Intravenous rtPA in Acute Ischemic Stroke
- Inclusion criteria
- Diagnosis of ischemic stroke causing measurable neurological deficit
- Onset of symptoms < 3 hours before beginning treatment
- Aged ≥18 years
- Exclusion criteria
- Significant head trauma or prior stroke in previous 3 months
- Symptoms suggest subarachnoid hemorrhage
- Arterial puncture at noncompressible site in previous 7 days
- History of previous intracranial hemorrhage
- Intracranial neoplasm, arteriovenous malformation, or aneurysm
- Recent intracranial or intraspinal surgery
- Elevated blood pressure (systolic >185 mm Hg or diastolic >110 mm Hg)
- Active internal bleeding
- Acute bleeding diathesis, including but not limited to
- Platelet count <100 000/mm³
- Heparin received within 48 hours, resulting in elevated aPTT greater than the upper limit of normal
- Current use of anticoagulant with INR >1.7 or PT >15 seconds
- Current use of direct thrombin inhibitors or direct factor Xa inhibitors with elevated laboratory tests
- Blood glucose concentration <50 mg/dL (2.7 mmol/L)
- CT demonstrates multilobar infarction (hypodensity >1/3 cerebral hemisphere)
- Relative exclusion criteria:
- Only minor or rapidly improving stroke symptoms (clearing spontaneously)
- Pregnancy
- Seizure at onset with postictal residual neurological impairments
- Major surgery or serious trauma within previous 14 days
- Recent gastrointestinal or urinary tract hemorrhage (within previous 21 days)
- Recent acute myocardial infarction (within previous 3 months)
In addition, strict adherence to the NINDS recommended protocol for administration of rtPA is critical to successful treatment in stroke patients. This protocol specifies important aspects of care during and after administration of rtPA. Admission to an ICU or stroke unit, frequent reassessment of the patient’s neurologic status and careful blood pressure monitoring are all vital in the first 24 hours after administration of rtPA. Most importantly, any patient who develops acute severe headache, acute severe hypertension, intractable nausea and vomiting, altered mental status or other evidence of neurologic deterioration during or after rtPA administration should have emergent noncontrast head CT to evaluate for ICH. In addition, rtPA infusion should be discontinued immediately if it has not already been completed.
Alternative Therapies
Unfortunately, only a very small percentage of stroke patients present to the ED within the time limit for intravenous thrombolytic therapy. Selected patients presenting to a specialized stroke center greater than 4.5 hours after onset of symptoms may be eligible for intra-arterial thrombolytic therapy, mechanical thrombectomy or intracranial angioplasty and stenting in the setting of a clinical trial.
Supportive Care
In stroke patients not receiving rtPA or other specialized therapy, the goal of care is to prevent or treat acute complications by providing supportive care. This includes ventilatory support and oxygenation, prevention of hyperthermia, cardiac monitoring and treatment, and control of blood pressure and blood glucose.
Goals for Blood Pressure Control
In patients receiving intravenous rtPA, the rate of symptomatic ICH is directly related to increasing blood pressure. Therefore, strict guidelines for blood pressure control must be enforced in these patients to prevent ICH. Blood pressure should be maintained below 180/105 mm Hg in the first 24 hours after receiving thrombolytic therapy. In contrast, the ideal blood pressure range for acute stroke patients not receiving thrombolytic therapy has not yet been determined. The current recommendations stress the importance of an individualized approach to blood pressure control with avoidance of hypotension or large fluctuations in blood pressure. For patients who do not have other medical conditions requiring aggressive blood pressure control, antihypertensive treatment should not be initiated unless blood pressure exceeds 220/120 mm Hg.
Antiplatelet Therapy
Administration of Aspirin within 48 hours after stroke has been shown to improve outcomes by reducing the rate of early recurrent stroke. In stroke patients not receiving rtPA, oral administration of aspirin within 24 – 48 hours of stroke onset is recommended. The safety of antiplatelet agents in combination with thrombolytic therapy has not been established. Therefore, aspirin should not be administered for at least 24 hours after administration of rtPA.
Pearls and Pitfalls
- Use creative questioning to establish time of onset.
- Consider common stroke mimics including seizure, complicated migraine, hypoglycemia, and conversion disorder
- Know the exclusion criteria for thrombolytic therapy
- Minimum workup prior to thrombolytic therapy includes focused H&P, CT Head to exclude intracranial hemorrhage and Point of care blood glucose level to exclude hypoglycemia.
- Time is brain! Do not delay administration of thrombolytic therapy to eligible patients.
- Patients that do not receive thrombolytic therapy should receive aspirin within 24 hours of symptom onset
References
- Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Go AS, et al. Circulation. 2014 Jan 21;129(3):399-410. PMID:24446411
- Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Jauch EC, et al. Stroke. 2013 Mar;44(3):870-947. PMID:23370205
- Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lees KR, et al. Lancet. 2010 May 15;375(9727):1695-1703. PMID:20472172
- Safety of thrombolysis in stroke mimics: results from a multicenter cohort study. Zinkstok SM, et al. Stroke. 2013 Apr;44(4):1080-PMID:23444310
