Pelvic Inflammatory Disease and Tubo-Ovarian Abscess
Objectives
Upon finishing this module, the student will be able to:
- Recognize the signs and symptoms of Pelvic Inflammatory Disease (PID) and Tubo-Ovarian Abscess (TOA).
- Initiate appropriate diagnostic testing.
- Name the risk factors for PID.
- Discuss the complications of PID.
- Understand the rationale for initiating treatment for patients with potential PID in uncertain cases.
- Determine which women with PID require hospitalization.
Contributors
Update Author: Simi Jandu, MD.
Original Author: Collette Wyte, MD.
Update Editor: William Alley, MD.
Last Updated: November 2024
Introduction
Pelvic Inflammatory Disease (PID) in women is inflammation of the upper genital tract due to an infection. It is an acute clinical syndrome that frequently originates as a cervical infection ascending into the uterus, fallopian tube, and ovaries. It is most commonly diagnosed in females aged 15-25 years old. The most common causes are sexually transmitted diseases, where 85% are caused by Neisseria gonorrhea or Chlamydia trachomatis. Mycoplasma genitalium is also a common cause in pre-menopausal females.
PID remains a common and serious infection in reproductive-aged women in the United States with its prevalence being stable. There are approximately 90,000 visits in the United States per year for PID. 4.4% of sexually active women aged 18-44 years in the U.S. have been treated for PID in their lifetime. Risk factors of PID include:
- Having had sexual intercourse
- Multiple sexual partners
- Younger age
- Sexual partners with sexual transmitted infection (STI)
- History of PID and chlamydia
Protective factors include the use of barrier contraceptives. PID is primarily a clinical diagnosis and should be suspected in females presenting with abdominal and pelvic pain. Antibiotics targeting Neisseria gonorrhea and Chlamydia trachomatis are the treatment of choice and may prevent complications such as tubo-ovarian abscess (TOA). Additional complications include perihepatitis (Fitz-Hugh-Curtis syndrome), endometritis, salpingitis, oophoritis, and pelvic peritonitis. Long-term complications include chronic pelvic pain and tubal damage leading to an increased risk of ectopic pregnancy, infertility, and hydrosalpinx.
A 17-year-old female presents to the emergency department (ED) with five days of lower abdomen and pelvic pain that does not radiate. Her pain worsened over the past 24 hours with sexual intercourse. She denies any alleviating factors. She also noted foul-smelling vaginal discharge, post-coital bleeding, fevers, and chills. She is sexually active with three male partners. She also notes that she had an IUD placed two weeks ago. She denies nausea, vomiting, dysuria, or changes in her bowel movements. Her last menstrual period (LMP) was three weeks ago. She has no history of abdominal surgeries.
Her vital signs are the following: T 102F, BP 100/60, HR 120, RR 16. She appears to be in moderate distress due to pain. She has lower pelvic tenderness to palpation without rebound or guarding, and lower abdominal tenderness to palpation. On her pelvic exam, she has cervical motion tenderness with foul-smelling purulent vaginal discharge and right adnexal tenderness. There is no adnexal tenderness, no blood in the vaginal vault, her uterus is non-tender and normal size. She has no vaginal lesions or lacerations.
Any female of reproductive age who presents with vaginal discharge, lower abdominal pain, or pelvic discomfort should be considered for PID. All patients should receive a pregnancy test to rule out ectopic pregnancy. PID is less common in pregnancy, and if it does occur it is most common in the first 12 weeks of pregnancy.
After vital signs and ABCs (airway, breathing, circulation) evaluation, the initial evaluation of a patient with suspected PID should proceed with a detailed history including risk factors. Important historical information includes sexual history, number of sexual partners, condom use, characterization of pain, and associated urinary and GI symptoms. IV access should also be established and labs including a pregnancy test should be sent.
Physical exam should include an abdominal and pelvic exam, with a bimanual exam to assess for cervical motion tenderness, uterine tenderness, and adnexal tenderness characteristic of PID. A speculum exam should also be performed to evaluate for cervical mucopurulent discharge. A palpable adnexal mass or fullness is concerning for TOA.
The classic presentation for PID is a sexually active female aged 15-25 years old with multiple sexual partners who presents with less than two weeks of lower abdominal pain. The pain is generally bilateral and occurs commonly during or shortly after menstruation. Post-coital or inter-menstrual dyspareunia or abnormal vaginal bleeding can be associated symptoms. Vaginal discharge and urinary frequency are also commonly associated with PID.
The classic physical exam includes lower abdominal tenderness. There may be rebound tenderness, fevers, and in severe cases, decreased bowel sounds. On a pelvic exam, there may be cervical motion tenderness or uterine or adnexal tenderness, along with purulent vaginal or cervical discharge. A palpable adnexal mass is concerning for TOA. Right upper quadrant abdominal tenderness should elicit concern for perihepatitis (Fitz-Hugh Curtis syndrome).
Testing for all patients with presentations suspicious of PID should include a pregnancy test first to rule out ectopic pregnancy. Additional labs include urinalysis, CBC with differential, and liver function studies (if Fitz-Hugh–Curtis syndrome is suspected). The most appropriate testing for Neisseria gonorrhoeae and Chlamydia trachomatis is by nucleic acid amplification and hybridization techniques, such as polymerase chain reaction (PCR) or DNA probes. These tests are more sensitive than culture, can be performed on cervical secretions or urine, and have a more rapid turnaround time. Microscopic evaluation of vaginal discharge is also available for bacterial vaginosis and trichomoniasis. A gram stain of cervical secretions showing gram-negative intracellular diplococci is indicative of N. gonorrhoeae. Testing for other STIs, including HIV and syphilis, should also be considered. For patients with severe allergies to currently recommended antibiotics, cultures with sensitivity testing on cervical secretions may be necessary.
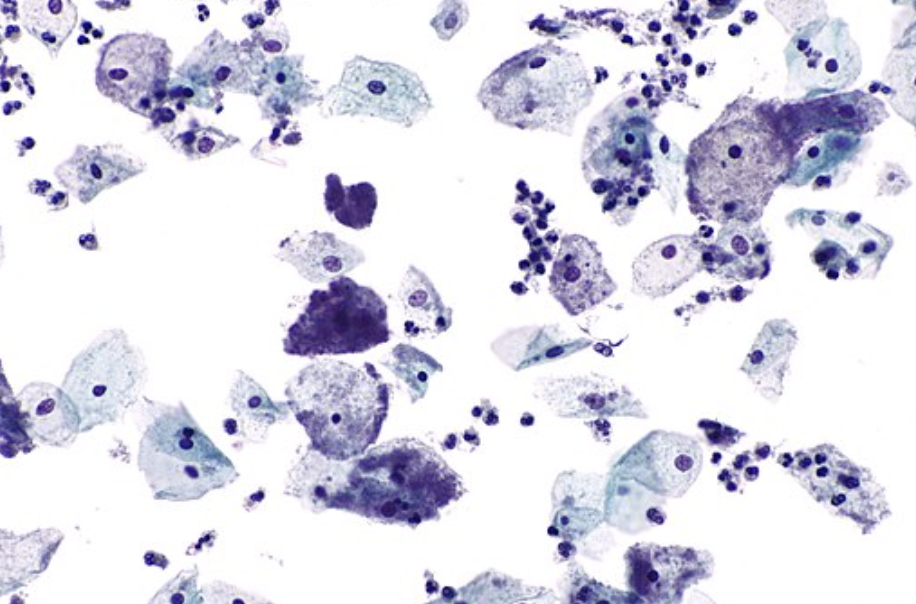
Figure: Clue cells - pap test. Wikimedia Commons. Published January 6, 2011. Image used under the Creative Commons Attribution-Share Alike 3.0 Unported license.

Figure: Right adnexa showed a complex cystic mass consistent with TOA. Radiopaedia. Image used under the Creative Commons Attribution-Share Alike 3.0 Unported license.
A pelvic ultrasound is warranted if a TOA is suspected or the diagnosis is unclear. It is particularly useful to rule out other diseases that may present with pelvic pain such as a ruptured ovarian cyst (free fluid in the pouch of Douglas) or ovarian torsion (absence of blood flow to one ovary on pelvic ultrasound with Doppler). The absence of radiographic findings does not rule out PID.
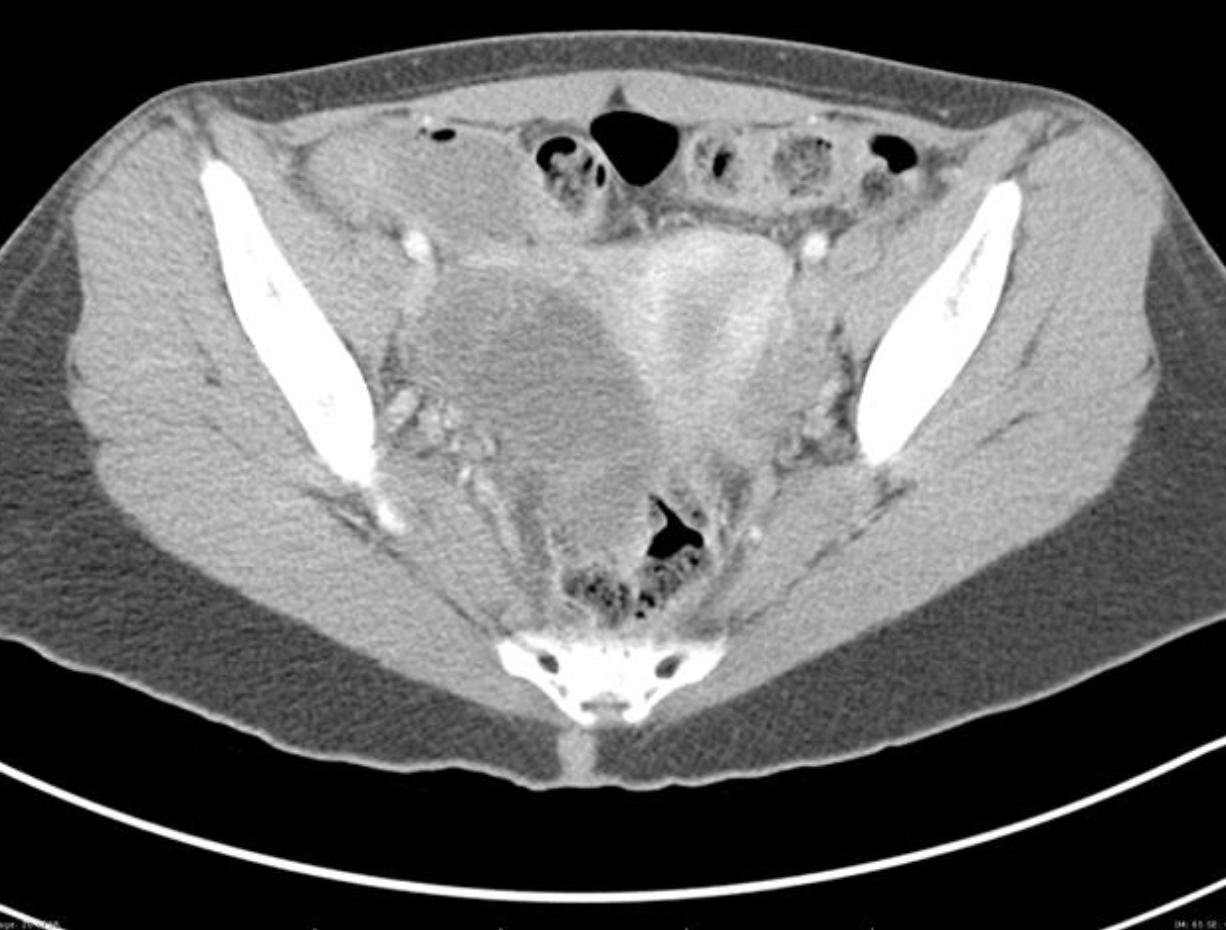
Figure: Complex mass into the anatomical space consistent with TOA. Radiopaedia. Image used under the Creative Commons Attribution-Share Alike 3.0 Unported license.
A CT of the abdomen/pelvis may also demonstrate TOA. It is not the preferred method of diagnosis due to radiation exposure and its inability to assess for other ovarian pathology (torsion). CT is frequently ordered in patients to evaluate for appendicitis or other suspected etiology and the scan may reveal a TOA instead. The CT scan above reveals a complex ovarian mass consistent with TOA.
How do I Make the Diagnosis?
In a patient with a negative pregnancy test and elevated WBC, in the setting of fever, cervical motion tenderness, and a mucopurulent cervical discharge, the diagnosis of PID is highly likely. A PCR positive for N. gonorrhea or C. trachomatis is suggestive for PID in the appropriate clinical setting but is not usually available while formulating the treatment plan in the ED. Up to one-third of women with PID do not have Neisseria or Chlamydia isolated, emphasizing the polymicrobial nature of this disease. Because of the potential complications of untreated PID and the prevalence of infection, the Centers for Disease Control and Prevention (CDC) recommends initiating empiric therapy for all patients who meet minimal clinical criteria for PID. This minimum criterion includes all of the following:
- A history of lower abdominal or pelvic pain.
- Adnexal, uterine, or cervical motion tenderness on exam.
- A patient at risk for STIs with no other discernible cause for the illness identified.
The complications of PID include chronic pelvic pain, dyspareunia, infertility, ectopic pregnancy, TOA, and Fitz-Hugh-Curtis syndrome. Women with a previous episode of PID are 12-15% more likely to have an ectopic pregnancy.
Tubo-Ovarian Abscess
TOAs are walled-off abscesses that originate in the infected fallopian tube and extend to involve the ovary. Women with TOA appear ill and will often have severe unilateral adnexal tenderness and fullness on bimanual pelvic examination. Fitz-Hugh-Curtis syndrome is a perihepatitis that occurs in 8-10% of patients with PID. These patients appear septic as well, and often present with right upper quadrant or referred right shoulder pain. Elevated liver function tests confirm the diagnosis.
Once the CDC minimal criteria is met, women should be treated with antibiotics that cover the multiple organisms potentially responsible for this disease, including N. gonorrhea, C. trachomatis, Escherichia coli, and multiple anaerobic bacteria such as Peptococcus, Peptostreptococcus, and Bacteroides. The particular agent used will be determined by the toxicity of the patient. Inpatient treatment should be started in the ED. The recommended regimen includes:
- Ceftriaxone 1g IV q 24 hours AND Doxycycline 100mg PO or IV q 12 hours AND metronidazole 500mg PO or IV q 12 hours, OR
- Cefoxitin 2g IV q 6 hours AND Doxycyline 100mg PO or IV q 12 hours, OR
- Cefotetan 2g q 12 hours AND Doxycycline 100mg PO or IV q 12 hours
If the patient is allergic to cephalosporins:
- Clindamycin 900mg IV q 8 hours AND Gentamycin 3mg/kg IV q daily or 2mg/kg once followed by 1.5mg/kg q 8 hours
- Ampicillin-sulbactam 3g IV q 6 hours AND Doxycycline 100mg IV or PO q 12 hours
Doxycycline should always be given orally when possible because it is caustic to vessels.
The recommended outpatient therapy is outlined below.

Disposition
Mild to moderate PID can be treated with outpatient treatment and follow-up. See below for a list of hospitalization criteria which include severity of PID, complications, pregnancy, inability to tolerate PO medication, lack of response or non-adherence, or requirement of intervention. Women with an IUD do not need to have it removed, but need to be followed closely for IUD removal if not clinically improving. Those with HIV are treated the same as the general population.
Hospitalization Criteria
| Criteria | Description |
| Severe clinical illness | Fever > 38.5C, vomiting, physician discretion |
| Complications of PID | Pelvic abscess (TOA), Fitz-Hugh-Curtis syndrome, pelvic peritonitis |
| Possible invasive diagnosis evaluation for alternative etiology or surgical intervention for ruptured TOA | TOA |
| Inability to tolerate oral medication regimen | |
| Pregnancy | |
| Lack of response to oral medication | |
| Concern for non-adherence to therapy |
Discharged patients should be advised to avoid sexual contact, to refer their partners for treatment, and follow up in 72 hours unless their symptoms worsen and require earlier follow-up. Patients should also be referred for further STD testing including HIV, hepatitis, and syphilis.
- Pelvic Inflammatory Disease is a polymicrobial infection despite being most commonly associated with Chlamydia and Gonorrhea.
- Women of reproductive age who present with pelvic pain require a pregnancy test. In the pregnant patient, PID is less likely (particularly after the first 12 weeks), and other causes such as ectopic pregnancy need to be excluded.
- Consider TOA or an alternative diagnosis if the patient does not improve with antibiotics.
- Patients with minimal CDC criteria should be treated for PID if no other discernible cause for their illness is identified to reduce complications associated with untreated PID.
- Pelvic ultrasound should be obtained in patients with unilateral tenderness or mass, or if the diagnosis is unclear.
- Partners of the patient should be seen and treated.
- Chomvarin C, Chantarsuk Y, et al. An Assessment and Evaluation of Methods for Diagnosis of Chlamydia and Gonococcal Infections. Southeast Asian J Trop Med Public Health. 1997 Dec.
- Dicker LW, Mosure DJ, et al. Testing for Sexually Transmitted Diseases in U.S. Public Health Laboratories in 2004. Sex Transm Dis. 2007.
- Jennings LK, Krywko DM. Pelvic Inflammatory Disease. 2018.
- Kreisel K. Prevalence of Pelvic Inflammatory Disease in Sexually Experienced Women of Reproductive Age - United States, 2013-2014. 2017.
- Mitchell CM, Anyalechi GE, et al. Etiology and Diagnosis of Pelvic Inflammatory Disease: Looking Beyond Gonorrhea and Chlamydia. J Infect Dis. 2021.
- Newman L, Moran JS, Workowski KA. Update on the Management of Gonorrhea in Adults in the United States. Clin Infect Dis. 2007.
- Pelvic Inflammatory Disease (PID). Centers for Disease Control and Prevention. Updated 2022.
- Ross J. Pelvic Inflammatory Disease: Pathogenesis, Microbiology, and Risk Factors. 2016.
- Soper DE. Pelvic Inflammatory Disease. Obstet Gynecol. 2010.
- Wiesenfeld HC. Pelvic Inflammatory Disease: Treatment in Adults and Adolescents. 2019.
